Introduction:
First degree relatives of patients with monoclonal gammopathy of undetermined significance (MGUS) or multiple myeloma (MM), and African Americans, have an increased risk of having MGUS and MM compared to the general population. However, current guidelines do not advocate for screening family members of MM for plasma cell disorders (PCD). Understanding the epidemiology of familial plasma cell disorders (PCDs) is essential in order to identify people at highest risk of developing PCDs who may benefit from targeted screening strategies. The aims of this study were to assess the prevalence of MM patients with a family history of PCD, and the implications of a family history of PCD on the overall survival (OS) of MM patients.
Methods:
We retrospectively reviewed the electronic medical records of patients with symptomatic MM followed at Mayo Clinic and diagnosed between January 1989 and June 2019. Clinical notes were reviewed for documentation pertaining to family history of PCDs. Kaplan Meier survival analysis was used to assess the OS, where OS was calculated from the date of diagnosis of symptomatic MM until death; patients were censored if they were alive at the date of last follow up. A Cox proportional hazards model was used to provide risk estimates for OS.
Results:
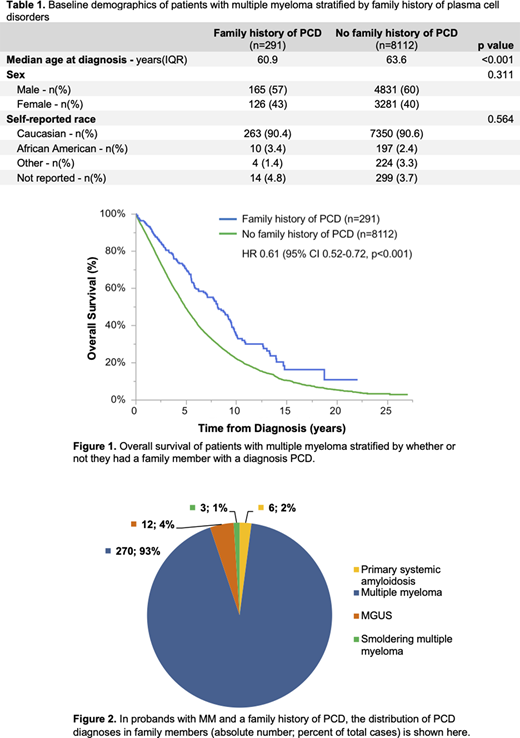
A total of 8403 patients with symptomatic MM were included in this study. Family history was documented in 1521 patients, and 291 patients (3.5% of all patients, 19% of those with any documented family history) had a documented family member with a PCD. The median age at diagnosis of symptomatic MM was significantly lower in patients with (n=291) versus without (n= 8112) a family history of PCD (60.9 versus 63.6 years, p<0.0001). The median OS of MM patients with a family history of PCD was significantly longer than MM patients without a family history of PCD (8.1 versus 4.9 years, respectively, with p<0.0001, see figure 1). Using a multivariable Cox proportional hazards model, MM patients with a family history of PCD had a significantly lower risk of death compared to those without a family history of PCD (HR 0.66, 95% CI 0.55-0.78, p<0.0001) even after adjusting for sex, age at diagnosis (above versus below age 65), self-reported race (African American versus not African American), or date of diagnosis (before versus after 2010). When restricting the analyses to the 1521 patients with clearly documented family history, the survival benefit amongst patients with versus without a family history of PCD was similar.
In probands with a PCD family history, 182 (63%) had a first degree relative with PCD, whereas 109 (37%) had a second degree relative with PCD. The most common reported PCD amongst family members was MM (figure 2). Nineteen (6.5%) probands with a family history of PCD had 2 or more relatives with a PCD (6 MM patients had 2 or more first degree relatives, 5 MM patients had at least 1 first and 1 second degree relative, and 8 MM patients had 2 or more second degree relatives with a PCD history). There was no significant difference in the median OS between MM patients with a first degree versus second degree relative with PCD (HR 1.03, 95% CI 0.74-1.46, p=0.837), or MM patients with 1 versus 2 or more relatives with PCD (HR 1.01, 95% CI 0.53-1.96, p=0.962).
Conclusion:
We reviewed patients with symptomatic MM seen at Mayo Clinic over the last 30 years and found that the prevalence of patients with a documented family history of PCD was 3.5%. MM patients with a family history of PCD were diagnosed with MM at a younger age and survived longer than patients without a family history of PCD. Further work is needed to understand factors underlying the survival benefit in patients with a family history of PCD, and whether they present with less aggressive or less advanced disease at diagnosis.
Dispenzieri:Intellia: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Janssen: Research Funding. Kapoor:Sanofi: Consultancy, Research Funding; Cellectar: Consultancy; Janssen: Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Celgene: Honoraria. Gertz:Spectrum: Other: personal fee, Research Funding; Janssen: Other: personal fee; Abbvie: Other; Amgen: Other: personal fee; Physicians Education Resource: Other: personal fee; Medscape: Other: personal fee, Speakers Bureau; Prothena: Other: personal fee; Teva: Speakers Bureau; Celgene: Other; Research to Practice: Other; Sanofi: Other; DAVA oncology: Speakers Bureau; Annexon: Other: personal fee; Appellis: Other: personal fee; Ionis/Akcea: Other: personal fee; Alnylam: Other: personal fee; Aurora Bio: Other; Proclara: Other; Johnson and Johnson: Speakers Bureau; Springer Publishing: Patents & Royalties. Kumar:Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Novartis: Research Funding; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Carsgen: Other, Research Funding; Cellectar: Other; Dr. Reddy's Laboratories: Honoraria; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; BMS: Consultancy, Research Funding; Karyopharm: Consultancy; MedImmune: Research Funding; Sanofi: Research Funding; Tenebio: Other, Research Funding; Kite Pharma: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Genecentrix: Consultancy; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Merck: Consultancy, Research Funding; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal